Archive for September, 2010
Dr Spock – Activate my Telomerase
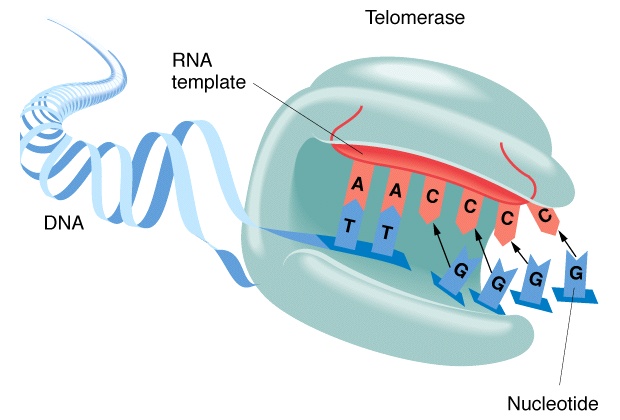
As you should know telomeres are the end caps on your chromosomes that break down over time.
This is how nature kills us through natural aging. Other factors can cause cellular damage but it is the breakdown of the telomeres that is the fundamental aging mechanism.
The body uses an enzyme called telomerase to repair the telomeres – all of this is old news (no pun intended).
So what we want to do is activate the telomerase enzyme to repair our healthy cells and prolong life – potentially indefinitely with this biotechnology.
Certain enzymes inhibit telomerase in such a way that destroys cancer cells & others activate it in such a way that restores healthy cells that we don’t want to age. The aging in the cells can be reversed by restoring the telomeres.
OK like I said this is old news, now products exist that do this. Cycloastragenol is a saponin from the Chinese herb astragalus – not just any astragalus though. TA-65, is the telomerase activator agent derived from the Chinese astragalus plant.
Geron corporation is distributing this product under license. http://www.tasciences.com/ are the original developers of the substance. Unfortunately as is often the case just taking astragalus is not going to save you as you need piles of the stuff to extract a daily dose.
Telomerase activation does hold the key to biological life extension.
Myostatin drug breakthroughs
It seems those myostatin blocking drugs are not far off. They will be beneficial in fighting cancer, aging and enhancing performance.
From http://www.acceleronpharma.com/content/products/ace-031.jsp
ACE-031 (Neuromuscular Disease)
ACE-031 is a novel, muscle-building agent that is being developed for the treatment of patients with neuromuscular diseases with the goal of improving strength and preserving physical function.
What is ACE-031?
ACE-031 is an investigational protein therapeutic that builds muscle and increases strength by inhibiting molecules that bind to and signal through a cell surface receptor called Activin Receptor Type IIB (ActRIIB). ACE-031 is a recombinant fusion protein that is produced by joining a portion of the human ActRIIB receptor to a portion of a human antibody. This creates a freely circulating, decoy version of ActRIIB which removes proteins, such as GDF-8 (myostatin) and other related molecules that limit the growth and strength of muscle.
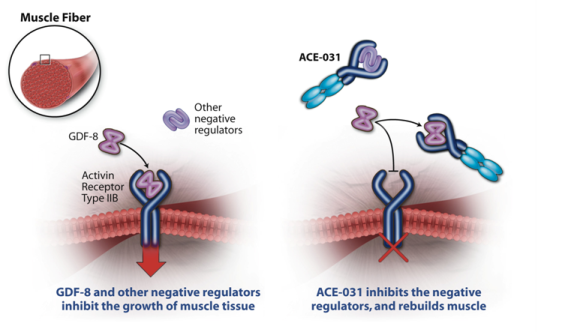
The Role of ActRIIB Signaling and Muscle Growth
Muscle growth is regulated by proteins in the TGF-? protein superfamily that serve as “on” or “off” switches for muscle production. Several molecules including GDF-8 interact with the ActRIIB receptor and send an “off” signal to stop muscle production. In the absence of these “off” switch molecules that signal through the ActRIIB receptor, muscle mass increases dramatically.
In nature, this effect has been observed in numerous species, particularly in animals that have been bred for increased musculature and strength. For example, Belgian Blue cattle lack the gene for GDF-8, which is one of several molecules that activate the ActRIIB receptor. A deficiency of this protein results in cattle with tremendously developed musculature and strength. Similar effects have been observed in other species, including rodents, dogs and even humans.
Treatment with ACE-031 Builds Skeletal Muscle
Treatment with ACE-031 promotes muscle growth by inhibiting ActRIIB signaling. ACE-031 binds to proteins that signal through the ActRIIB receptor to limit muscle growth. When ACE-031 binds to these proteins, it prevents them from interacting with the ActRIIB receptor, thus allowing muscle to grow. Moreover, because ACE-031 prevents GDF-8 and other proteins that regulate muscle mass from signaling through the ActRIIB receptor, its effects on lean muscle exceed those of inhibitors of GDF-8 (myostatin) alone.
Non-Clinical Results – When animals are treated with ACE-031, they experience growth in lean muscle and are considerably stronger than their untreated counterparts. This has been shown in several species, and in both healthy animals and in animals with diseases associated with muscle weakness and wasting.
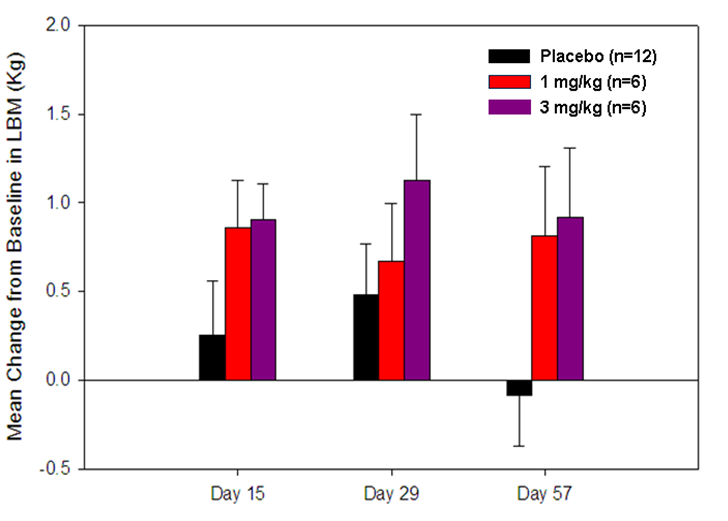
Clinical Results – Recently, Acceleron completed a clinical study of ACE-031 in healthy volunteers. These subjects received a single dose of ACE-031 across a range of dose levels. At higher doses, the effects of ACE-031 on skeletal muscle were encouraging. After a single dose of ACE-031, subjects developed roughly 1 kilogram (over 2 pounds) of muscle at 2 weeks. Moreover, ACE-031 was well-tolerated at all dose levels, with only mild or transient side effects observed.
Effects of Single-Dose Treatment of ACE-031 on Lean Mass (Human)
Acceleron is developing ACE-031 for the treatment of patients with neuromuscular diseases, such as Duchenne Muscular Dystrophy (DMD) and Amyotrophic Lateral Sclerosis (ALS), with the goal of improving strength and preserving physical function. By affecting the muscle directly, ACE-031 may one day offer hope to patients suffering from these debilitating diseases.
References
A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs, Mosher DS et al. PLoS Genet 3(5): e79, 2007.
Regulation of muscle growth by multiple ligands signaling through activin type II receptors, Lee SJ et. al., PNAS 102:18117-18122, 2005.
Inhibition of myostatin in adult mice increases skeletal muscle mass and strength, Whittemore LA et al., Biochem Biophys Res Commun. 2003 Jan 24;300(4):965-71.
Regulation of myostatin activity and muscle growth, Lee SJ et. al., PNAS, 98:9306-9311, 2001.
From http://www.nature.com/news/2010/100819/full/news.2010.419.html
Drug flexes muscle against cancer
Decoy protein helps to fight cancer in mice by stopping muscle breakdown.
Researchers have created a molecule that, in mice, can fully reverse the devastating muscle loss that often accompanies advanced cancer — and thereby increase the lifespan of animals with the disease.
The molecule blocks the activity of a key muscle-limiting protein called myostatin by acting as a decoy. Instead of myostatin binding to its normal receptor and triggering muscle wastage, it is ‘mopped up’ by binding to the decoy molecule instead.
Muscle wasting — called cachexia — is thought to account for about 30% of deaths in patients with cancer, but how exactly cachexia is spurred by cancer — or indeed exactly how it leads to a patient’s decline — isn’t known. It is thought that several molecular pathways work in tandem, “activating an axis of evil to control muscle mass in a negative way”, says H. Q. Han, lead author of the study and scientific director of the metabolic disorders division at Amgen, a biotechnology company in Thousand Oaks, California.
Han and his group wanted to find the dominant pathway responsible for cancer cachexia, and then design a way to block it in order to treat patients. Several studies have shown that blocking the myostatin pathway can promote muscle growth, says Han, and some have shown that a molecule closely related to myostatin, called activin A, becomes more abundant in patients with some cancers.
“We examined a large random collection of cancer cell lines in vitro, and found that one-third of them secreted large amounts of activin A,” says Han. “This led us to believe that activin A must have some systemic function when overproduced in a cancer setting.”
Muscling in
The researchers created a soluble version of the activin A receptor — which is thought to affect both myostatin and activin A signalling — by fusing a piece of human activin receptor to an antibody. This decoy mopped up the ligands that usually bind to the real receptor, thus blocking receptor activation.
“There’s really an overwhelming amount of data now showing the benefits of targeting this pathway.”
A single injection of the soluble receptor into normal mice boosted their muscle mass by 25% or more in a week or two. When it was given to mice implanted with colon cancer cells, their muscle mass returned to normal, even though their tumours continued to grow. Strikingly, all of the animals that did not receive the soluble receptor were dead 40 days after cancer cells were implanted, but more than half of the treated animals survived to this point. The study will be published tomorrow in the journal Cell1.
Han’s group isn’t the first to try to manipulate the myostatin pathway to treat muscle wasting. “There’s really an overwhelming amount of data now showing the benefits of targeting this pathway,” says Se-Jin Lee, a molecular biologist at Johns Hopkins University in Baltimore, Maryland, who co-discovered the myostatin gene and its role in regulating skeletal muscle mass in 19972.
The fact that disrupting the myostatin pathway caused such strong muscle regrowth isn’t so surprising, says Lee, because other studies have shown that this pathway has an extremely negative effect on muscle growth.
Ken Fearon, a surgical oncologist at the University of Edinburgh, UK, who has studied cancer cachexia, agrees. “They’ve antagonized one of the main in vivo brakes” to muscle growth, he says. “If you take the brakes off a car, it’ll keep going down the road.”
Limited options
What is most exciting is that the treatment prolonged survival, according to Lee and Fearon, because few treatments for cancer cachexia currently exist. “The reason that oncologists don’t bother measuring — let alone treating — cachexia is that they feel their options are so limited outside of treating the cancer,” Fearon says.
Despite the molecule’s powerful effect in mice, “does blocking this pathway in humans also cause muscle to grow?” asks Lee. “That question has not been answered yet.”
It is also still unclear whether the myostatin pathway plays a causative role in regulating the condition, he says, and the study doesn’t provide all the answers. “The fact that you can prevent the muscle loss doesn’t say that it’s due to overactivity in this pathway,” he notes.
Several pharmaceutical and biotech companies have begun clinical trials to test compounds that target the myostatin pathway. In the only such study published so far, researchers from Johns Hopkins University and drug firm Wyeth (now part of Pfizer) used an antibody to block myostatin in an attempt to treat the muscle-wasting disease muscular dystrophy3. “Those results were quite wishy-washy,” Lee says.
Other trials, including on testing a similar form of the soluble activin A receptor by pharmaceutical company Acceleron, based in Cambridge, Massachusetts, are still in progress. Unlike many of the other compounds being tested, says Lee, this particular compound can bind not just to myostatin and activin but also to many other related molecules. This could make it more potent, he notes, “but of course it might also be the downfall” if this lack of specificity leads to unwanted side effects in patients.
*
References
1. Zhou, X. et al. Cell 142, 531-543 (2010).
2. McPherron, A. C. et al. Nature 387, 83-90 (1997). | Article
3. Wagner, K. R. et al. Ann. Neurol. 63, 561-571 (2008). | Article